Health
Neuro Event Labs Secures FDA Clearance for Innovative Seizure Monitor
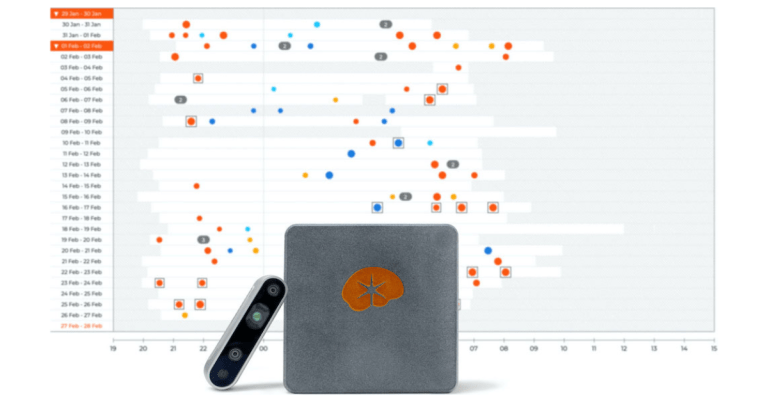
Neuro Event Labs Oy has received clearance from the U.S. Food and Drug Administration (FDA) for its Nelli Seizure Monitoring System, marking a significant advancement in epilepsy diagnostics. This system, recognized as a Breakthrough Device by the FDA, is the world’s first non-contact, AI-driven video and audio monitoring solution designed to effectively identify and prioritize the review of seizure events that exhibit positive motor components.
With this FDA clearance, Nelli is set to enhance clinical practices in U.S. hospitals, providing a new tool for improving epilepsy diagnostics and expanding monitoring capacity. The platform offers objective and advanced measurement capabilities for documenting seizure outcomes, particularly in clinical trials focusing on anti-seizure medications.
Innovative Features and Clinical Validation
According to Kaapo Annala, Founder and Chief Operating Officer at Neuro Event Labs, “Nelli’s AI-based motor seizure detection helps hospitals expand monitoring capability without adding staff burden.” The non-contact design and seamless integration into existing epilepsy monitoring workflows enable hospitals to enhance safety and diagnostic monitoring capacity, particularly in high-demand clinical environments.
The FDA’s clearance follows a pivotal validation study conducted in collaboration with Thomas Jefferson University Hospital. Principal Investigator Michael Sperling, MD, who is also the Baldwin Keyes Professor of Neurology and Director of the Jefferson Comprehensive Epilepsy Center, stated, “We’ve found Nelli to be highly sensitive and specific for detecting and classifying motor movements when compared to gold-standard video-EEG monitoring.” He emphasized that this technology complements existing diagnostic modalities and significantly broadens monitoring capabilities.
Positive Reception from the Medical Community
The epilepsy field has expressed strong support for innovations that enhance patient care. Jacqueline French, MD, Medical Director of the Epilepsy Foundation and Professor of Neurology at NYU Langone Health’s Comprehensive Epilepsy Center, remarked, “The epilepsy field welcomes technologies that enhance patient care and allow more efficient use of EMU resources.”
Neuro Event Labs’ Martijn Wallert, CEO, shared his enthusiasm for this milestone, stating, “This milestone marks a new chapter for Neuro Event Labs. Nelli gives hospitals a scalable monitoring solution that immediately supports staff and strengthens diagnostic workflows.” The company anticipates bringing Nelli to leading epilepsy centers across the United States, with a commercial rollout planned to commence in early 2026. Priority will be given to major academic hospitals and high-volume epilepsy centers as they integrate this innovative technology into their clinical practices.
-

 Entertainment3 months ago
Entertainment3 months agoAnn Ming Reflects on ITV’s ‘I Fought the Law’ Drama
-

 Entertainment4 months ago
Entertainment4 months agoKate Garraway Sells £2 Million Home Amid Financial Struggles
-

 Health3 months ago
Health3 months agoKatie Price Faces New Health Concerns After Cancer Symptoms Resurface
-

 Entertainment3 months ago
Entertainment3 months agoCoronation Street’s Carl Webster Faces Trouble with New Affairs
-

 Entertainment3 months ago
Entertainment3 months agoWhere is Tinder Swindler Simon Leviev? Latest Updates Revealed
-

 Entertainment2 weeks ago
Entertainment2 weeks agoCoronation Street Fans React as Todd Faces Heartbreaking Choice
-

 World2 weeks ago
World2 weeks agoBailey Announces Heartbreaking Split from Rebecca After Reunion
-

 Entertainment4 months ago
Entertainment4 months agoMarkiplier Addresses AI Controversy During Livestream Response
-

 Science1 month ago
Science1 month agoBrian Cox Addresses Claims of Alien Probe in 3I/ATLAS Discovery
-

 Health5 months ago
Health5 months agoCarol Vorderman Reflects on Health Scare and Family Support
-

 Entertainment4 months ago
Entertainment4 months agoKim Cattrall Posts Cryptic Message After HBO’s Sequel Cancellation
-

 Entertainment3 months ago
Entertainment3 months agoOlivia Attwood Opens Up About Fallout with Former Best Friend