Science
Researchers Confirm First Dynamic Transition in Supercooled Water
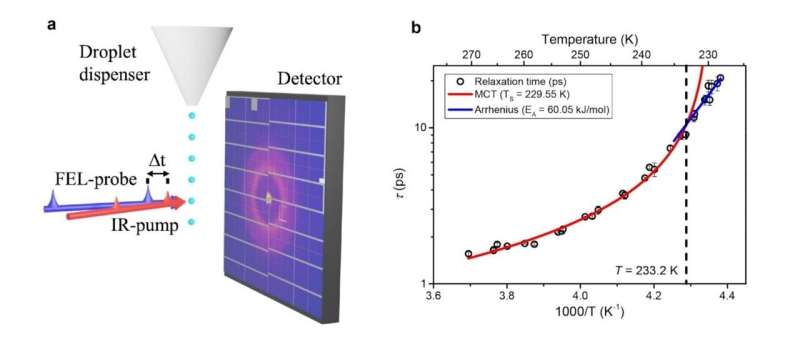
A groundbreaking study published in Nature Physics has marked the first experimental observation of a fragile-to-strong transition in deeply supercooled water. This finding resolves a scientific challenge that has persisted for nearly three decades, further illuminating the anomalous behavior of this essential substance.
Water exhibits unique properties when cooled below its freezing point without crystallization. Previous research indicated that the viscosity of water would diverge to infinity around 227 K (approximately –46°C), suggesting that liquid water’s motion would effectively freeze. Yet, this prediction conflicted with other known characteristics of water, leading scientists to propose that the viscosity trend undergoes a significant change at a specific low temperature.
To delve deeper into this phenomenon, researchers approached the study with insights from both theory and experimentation. Co-authors of the study, Prof. Kyung Hwan Kim from POSTECH and Prof. Anders Nilsson from Stockholm University, discussed the importance of their work. “Water is the most essential substance for life and for countless natural phenomena, yet it is also the liquid that exhibits the most anomalous properties,” noted Kim. “The key to uncovering their origin is believed to lie in the deeply supercooled regime.”
Overcoming Experimental Challenges
The fragile-to-strong transition had eluded direct experimental verification primarily due to the rapid crystallization of water when cooled below approximately 235 K. The researchers’ breakthrough involved an innovative approach that combined a droplet-based sample scheme with ultrafast X-ray free-electron lasers.
“We created liquid water down to –45°C by rapidly evaporating it under vacuum, allowing it to remain supercooled for only a brief moment,” explained Nilsson. The experimental setup generated micron-sized water droplets, about 17 μm across, which traveled through a vacuum chamber. As the droplets evaporated, they reached deeply supercooled temperatures between 228 K and 270 K (or –45°C to –3°C).
The researchers then applied femtosecond infrared laser pulses to induce a small temperature change of less than 1 K in each droplet. They monitored the liquid structure’s relaxation back toward equilibrium, capturing how water’s hydrogen-bonding structure evolved after each temperature perturbation.
Tracking Structural Relaxation
Utilizing ultrashort X-ray pulses from the SwissFEL facility, the team observed wide-angle X-ray scattering patterns at various time delays. This allowed them to directly track the structural relaxation dynamics of water. “In our experiment, we applied a small perturbation to liquid water at each temperature and then tracked the structural relaxation dynamics to a new equilibrium state over time,” Kim stated.
The results revealed a clear dynamic crossover at approximately 233 K (around –40°C). Above this temperature, relaxation times increased rapidly with decreasing temperature, indicative of fragile liquids. In contrast, below 233 K, the data followed a shallower Arrhenius temperature dependence typical of strong liquids.
To validate their experimental findings, the researchers conducted molecular dynamics simulations using the TIP4P/2005 water model. This model successfully reproduced a similar fragile-to-strong crossover, with a transition appearing around 238.7 K.
The experimentally observed transition temperature of about 233 K is slightly above the previously identified Widom line at 230 K, suggesting a connection between the fragile-to-strong transition and changes in molecular arrangements rather than the glass transition at 136 K.
Implications and Future Directions
Understanding water’s dynamics is crucial for various scientific fields, from meteorology to biological chemistry. By elucidating the mechanisms behind water’s anomalous behavior, researchers hope to enhance our comprehension of numerous phenomena reliant on water.
“This work opens up new experimental territory,” Kim remarked. “We have not yet directly observed the detailed microscopic mechanisms behind this behavior. With further improvements, we believe it will be possible to probe these underlying mechanisms experimentally.”
The study confirms that water’s apparent divergence is interrupted by a genuine change in relaxation behavior, reinforcing decades of theoretical and simulation work on the fragile-to-strong crossover. As researchers continue to explore the complexities of water at low temperatures, they anticipate that new discoveries will emerge, expanding our understanding of this vital substance.
-

 World6 days ago
World6 days agoCoronation Street’s Shocking Murder Twist Reveals Family Secrets
-

 Entertainment5 months ago
Entertainment5 months agoKate Garraway Sells £2 Million Home Amid Financial Struggles
-

 Entertainment3 days ago
Entertainment3 days agoAndrew Pierce Confirms Departure from ITV’s Good Morning Britain
-

 Entertainment3 months ago
Entertainment3 months agoAnn Ming Reflects on ITV’s ‘I Fought the Law’ Drama
-

 Health3 months ago
Health3 months agoKatie Price Faces New Health Concerns After Cancer Symptoms Resurface
-

 Entertainment4 weeks ago
Entertainment4 weeks agoCoronation Street Fans React as Todd Faces Heartbreaking Choice
-

 World4 weeks ago
World4 weeks agoBailey Announces Heartbreaking Split from Rebecca After Reunion
-

 Entertainment1 week ago
Entertainment1 week agoTwo Stars Evicted from I’m A Celebrity Just Days Before Finale
-

 World1 week ago
World1 week agoKevin Sinfield Exceeds Fundraising Goal Ahead of Final Marathons
-

 Entertainment3 months ago
Entertainment3 months agoCoronation Street’s Carl Webster Faces Trouble with New Affairs
-

 Entertainment3 months ago
Entertainment3 months agoWhere is Tinder Swindler Simon Leviev? Latest Updates Revealed
-

 Entertainment4 months ago
Entertainment4 months agoMarkiplier Addresses AI Controversy During Livestream Response









