Science
Amsterdam Physicists Revolutionize Strontium Measurements with Rubidium
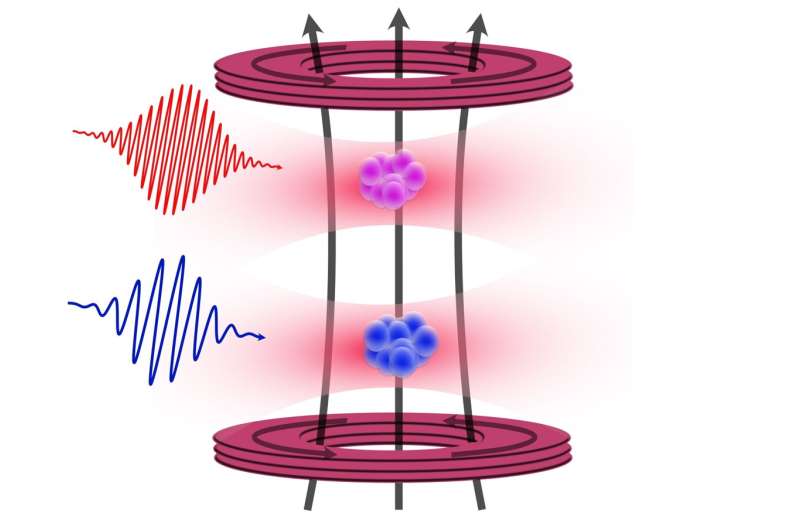
A team of physicists from the University of Amsterdam has achieved a groundbreaking advancement in the precision of measuring strontium atoms, a significant leap for applications in atomic clocks and quantum computing. This enhancement was made possible through the innovative use of nearby rubidium atoms, as detailed in their recent publication in the journal Physical Review Letters on November 4, 2025.
Strontium, while not widely recognized outside scientific circles, holds substantial importance among physicists. It is categorized as one of six alkaline earth metals, sharing characteristics with other elements such as magnesium and calcium. Strontium-87, or 87 Sr, is particularly notable due to its unique properties derived from its nuclear spin, distinguishing it from other isotopes of strontium. Unlike its even-numbered counterparts, which behave as bosons, 87 Sr functions as a fermion, giving it traits that are paramount for next-generation technologies.
Atomic clocks, especially optical clocks, depend on the precise frequencies of light that atoms emit or absorb. For 87 Sr, the ideal light frequency corresponds to red light at a wavelength of 698 nanometers. The fermionic nature of this isotope allows it to bypass the constraints faced by bosonic isotopes, thereby enabling effective transitions necessary for accurate timekeeping.
The research team, led by Premjith Thekkeppatt, focused on understanding the g-factor of 87 Sr, a critical parameter that determines how the energy levels of the nucleus split in a magnetic field. This splitting is essential for both atomic clocks and quantum computers, as it enhances the precision of measurements.
To achieve their results, the physicists employed a technique known as optical trapping to position 87 Sr in proximity to rubidium atoms. This configuration, although initially aimed at creating rubidium-strontium molecules, proved beneficial for measuring the g-factor through nuclear magnetic resonance. By leveraging the well-established properties of rubidium, they could accurately calibrate the magnetic field, leading to a hundredfold improvement over previous measurements of the g-factor.
The implications of this research extend beyond atomic clocks. The improved precision in measuring the g-factor sets a new benchmark for atomic structure calculations. Thekkepatt notes that their findings could inspire further advancements across various atomic species and states, potentially benefiting a wide range of applications.
In summary, the collaboration between strontium and rubidium has not only enhanced the understanding of 87 Sr but also paved the way for groundbreaking developments in quantum technology and precision measurement. This achievement highlights the significance of interdisciplinary approaches in scientific research and opens new avenues for future exploration in atomic physics.
-

 Entertainment2 months ago
Entertainment2 months agoAnn Ming Reflects on ITV’s ‘I Fought the Law’ Drama
-

 Entertainment3 months ago
Entertainment3 months agoKate Garraway Sells £2 Million Home Amid Financial Struggles
-

 Health2 months ago
Health2 months agoKatie Price Faces New Health Concerns After Cancer Symptoms Resurface
-

 Entertainment2 months ago
Entertainment2 months agoCoronation Street’s Carl Webster Faces Trouble with New Affairs
-

 Entertainment2 months ago
Entertainment2 months agoWhere is Tinder Swindler Simon Leviev? Latest Updates Revealed
-

 Entertainment3 months ago
Entertainment3 months agoKim Cattrall Posts Cryptic Message After HBO’s Sequel Cancellation
-

 Entertainment2 months ago
Entertainment2 months agoOlivia Attwood Opens Up About Fallout with Former Best Friend
-

 Science2 weeks ago
Science2 weeks agoBrian Cox Addresses Claims of Alien Probe in 3I/ATLAS Discovery
-

 Entertainment3 months ago
Entertainment3 months agoMarkiplier Addresses AI Controversy During Livestream Response
-

 Entertainment2 months ago
Entertainment2 months agoMasterChef Faces Turmoil as Tom Kerridge Withdraws from Hosting Role
-

 Entertainment4 months ago
Entertainment4 months agoSpeculation Surrounds Home and Away as Cast Departures Mount
-

 World2 months ago
World2 months agoCole Palmer’s Mysterious Message to Kobbie Mainoo Sparks Speculation