Top Stories
India Cuts GST on Nutraceuticals to 5% Amid Regulatory Changes

The Indian government has announced a significant reduction in the goods and services tax (GST) on nutraceuticals from 18% to 5%, effective from September 22, 2023. This decision aims to make health supplements more affordable for consumers. The Minister of Commerce has urged companies to ensure that these savings are passed on to their customers, potentially benefiting a wide range of products, including multivitamins, protein powders, and natural extracts.
This change is part of a broader initiative known as the “Next-Gen GST reforms,” introduced by the administration of Prime Minister Narendra Modi. The reduction aims to stimulate growth in the nutraceutical sector while encouraging consumers to make healthier choices.
U.S. FDA Proposals to Amend GRAS Regulations
In the United States, the Food and Drug Administration (FDA) is moving forward with proposals to amend its Generally Recognized as Safe (GRAS) regulations. Expected to be released in October, these proposals suggest an end to the self-affirmed GRAS pathway, a decision that could have significant implications for the food industry. Ashish Talati from Talati Law has indicated that the FDA’s intent to eliminate this pathway could exacerbate regulatory hurdles for companies.
This initiative follows a directive from Health and Human Services Secretary Robert F. Kennedy, Jr., who instructed the FDA to explore revisions to the current GRAS Final Rule. Critics, including Jonathan Emord, General Counsel for the Alliance for Natural Health, warn that eliminating self-affirmed GRAS could create substantial regulatory bottlenecks.
New Zealand’s Limited Exemptions for Supplement Exports
New Zealand has introduced exemptions from certain domestic labeling and composition rules for companies exporting dietary supplements and food, effective September 25, 2023. While this move is designed to ease export processes, Samantha Gray, the government affairs director at Natural Health Products NZ (NHPNZ), argues that the exemptions do not adequately address the industry’s primary challenge: the ability to make therapeutic claims. This limitation may hinder the potential growth of supplement exports from New Zealand.
China Tightens Regulations on Health Foods for Seniors
In China, the State Administration of Market Regulation (SAMR) is intensifying its oversight of health products marketed to seniors. This demographic has been increasingly targeted by unscrupulous marketing practices, often resulting in exorbitant prices for health foods. SAMR aims to protect senior consumers from misleading claims and high costs associated with these products.
In addition, SAMR announced new regulations that will require more comprehensive stability and safety reports for non-purified fermented raw materials. This regulatory shift is part of an effort to enhance consumer safety and promote responsible industry practices. The updates will be integrated into the existing “China Food Safety National Standard Good Health Food Production code (GB17405),” following a press conference held in late August.
The regulatory landscape for nutraceuticals and health foods is evolving rapidly across multiple countries. These shifts reflect a growing awareness of consumer protection and the need for transparent marketing practices in the health supplement industry.
-

 Entertainment1 month ago
Entertainment1 month agoKim Cattrall Posts Cryptic Message After HBO’s Sequel Cancellation
-

 Entertainment1 month ago
Entertainment1 month agoKate Garraway Sells £2 Million Home Amid Financial Struggles
-

 Entertainment3 weeks ago
Entertainment3 weeks agoMasterChef Faces Turmoil as Tom Kerridge Withdraws from Hosting Role
-

 Entertainment2 weeks ago
Entertainment2 weeks agoITV’s I Fought the Law: Unraveling the True Story Behind the Drama
-

 Entertainment1 month ago
Entertainment1 month agoAldi Launches Cozy Autumn Fragrance Range Ahead of Halloween
-

 Entertainment2 weeks ago
Entertainment2 weeks agoAnn Ming Reflects on ITV’s ‘I Fought the Law’ Drama
-

 Entertainment2 months ago
Entertainment2 months agoSpeculation Surrounds Home and Away as Cast Departures Mount
-

 Entertainment7 days ago
Entertainment7 days agoWhere is Tinder Swindler Simon Leviev? Latest Updates Revealed
-

 Entertainment1 month ago
Entertainment1 month agoMarkiplier Addresses AI Controversy During Livestream Response
-

 Lifestyle3 weeks ago
Lifestyle3 weeks agoSummer Flags Spark Controversy Across England as Patriotism Divides
-

 Health1 month ago
Health1 month agoWigan and Leigh Hospice Launches Major Charity Superstore
-
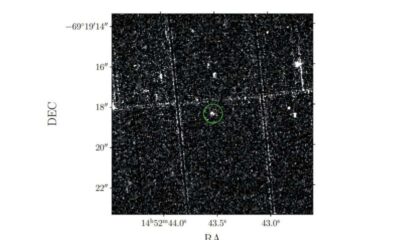
 Science1 month ago
Science1 month agoAstronomers Unveil New Long-Period Radio Transient ASKAP J1448−6856