Science
Researchers Uncover Process Behind Carbon’s Crystallization Choices
Researchers at the University of California, Davis have made significant strides in understanding how molten carbon determines its crystalline form, either becoming valuable diamond or more common graphite. Their study reveals the complex factors influencing this process, which has implications for various fields, including geology, nuclear fusion, and quantum computing. The findings were published in the journal Nature Communications.
Complex Behavior of Molten Carbon
At elevated temperatures and pressures, molten carbon faces a crucial decision: crystallize into diamond or graphite. While diamond is a highly sought-after material, graphite is more industrially useful. Understanding why molten carbon chooses one form over the other has proven challenging, particularly due to the rapid nature of crystallization and the extreme conditions required for experimentation.
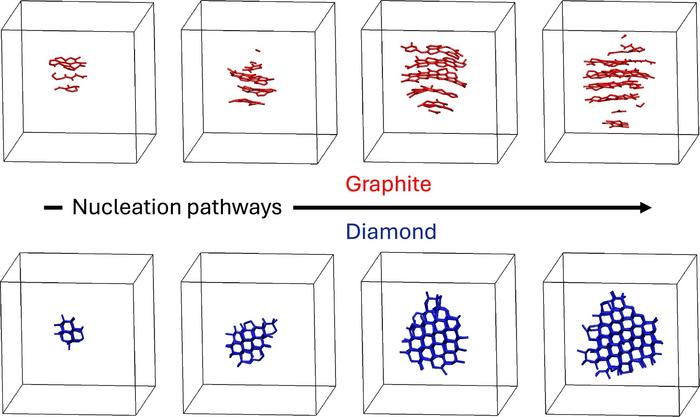
This research team, led by chemist Davide Donadio, utilized machine-learning-accelerated simulations to model how these crystalline forms emerge as liquid carbon cools from 5000 to 3000 K at pressures between 5 to 30 GPa. These conditions are difficult to replicate in a laboratory setting, often leading to conflicting results in previous studies.
The simulations indicated that crystallization behavior is more intricate than previously believed. Surprisingly, at lower pressures (up to 15 GPa), molten carbon tends to form graphite instead of diamond, despite diamond being the more thermodynamically stable phase under these conditions. This unexpected behavior aligns with an empirical observation known as Ostwald’s step rule, which suggests that crystallization typically progresses through intermediate metastable phases rather than directly to the most stable form.
Implications for Various Fields
According to co-author Tianshu Li, a professor of civil and environmental engineering at George Washington University, “The liquid carbon essentially finds it easier to become graphite first, even though diamond is ultimately more stable under these conditions. It’s nature taking the path of least resistance.” This insight could help clarify inconsistencies observed in earlier electrical and laser flash-heating experiments aimed at mapping carbon’s phase diagram near the graphite-diamond-liquid triple point.
Historical attempts to study this region often produced outcomes that suggested systems became “trapped” in metastable graphitic configurations. Understanding the process behind this entrapment could enhance manufacturing techniques for carbon-based materials, such as synthetic diamonds and nanodiamonds, which have applications in high-pressure and high-temperature environments.
Donadio expressed enthusiasm about the potential for future research, stating, “We will also be focusing on targeted pressures and temperatures, the likes of which are found in the interiors of giant planets in our solar system.” The team’s innovative approach using machine learning offers an effective method to explore the behavior of carbon, addressing challenges faced by traditional empirical and theoretical models.
This comprehensive understanding of molten carbon’s crystallization not only enriches scientific knowledge but also paves the way for advancements in technology and materials science, illustrating the interconnectedness of fundamental research and practical applications.
-

 World2 months ago
World2 months agoCoronation Street’s Shocking Murder Twist Reveals Family Secrets
-

 Entertainment2 months ago
Entertainment2 months agoAndrew Pierce Confirms Departure from ITV’s Good Morning Britain
-

 Health5 months ago
Health5 months agoKatie Price Faces New Health Concerns After Cancer Symptoms Resurface
-

 Health2 weeks ago
Health2 weeks agoSue Radford Reveals Weight Loss Journey, Shedding 12–13 kg
-

 Entertainment6 months ago
Entertainment6 months agoKate Garraway Sells £2 Million Home Amid Financial Struggles
-

 Entertainment5 months ago
Entertainment5 months agoAnn Ming Reflects on ITV’s ‘I Fought the Law’ Drama
-

 World3 months ago
World3 months agoBailey Announces Heartbreaking Split from Rebecca After Reunion
-

 Entertainment2 months ago
Entertainment2 months agoDavid Jason and Nicholas Lyndhurst Eye Reunion for Only Fools Anniversary
-

 Entertainment3 months ago
Entertainment3 months agoCoronation Street Fans React as Todd Faces Heartbreaking Choice
-

 World3 months ago
World3 months agoEastEnders’ Nicola Mitchell Faces Unexpected Pregnancy Crisis
-

 Entertainment2 months ago
Entertainment2 months agoBradley Walsh Sparks Strictly Come Dancing Hosting Speculation
-

 Entertainment2 months ago
Entertainment2 months agoTwo Stars Evicted from I’m A Celebrity Just Days Before Finale