Science
Magnetoelectric Nanotherapy Offers Hope for Pancreatic Cancer Patients
A groundbreaking study has revealed that magnetoelectric nanoparticles (MENPs) can effectively locate and destroy pancreatic tumors in preclinical models. This innovative approach, led by researchers from the Sylvester Comprehensive Cancer Center, the University of Miami College of Engineering, Moffitt Cancer Center, and Cellular Nanomed, Inc., presents a potential new method for treating one of the deadliest forms of cancer. The findings were published on November 3, 2025, in the journal Advanced Science.
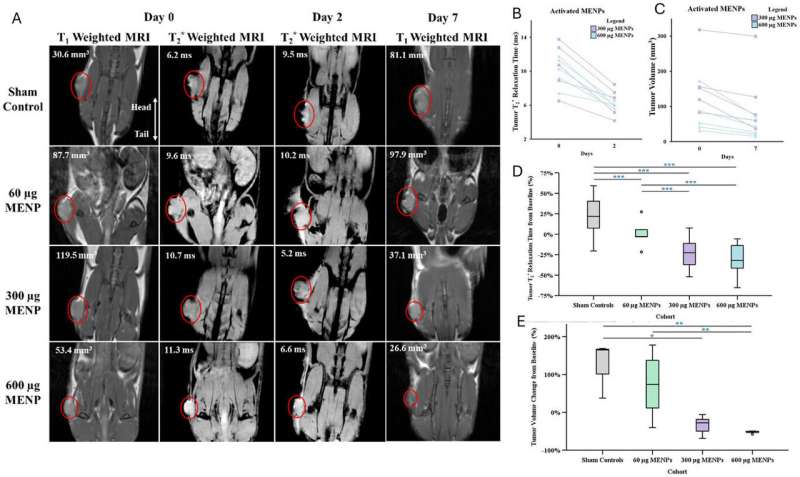
In the study, a single intravenous dose of MENPs, activated by a magnetic field within an MRI machine, resulted in pancreatic tumors shrinking to one-third their original size. Remarkably, one-third of the treated models showed complete tumor disappearance. This novel treatment also more than doubled survival time for subjects, all while sparing healthy organs from damage.
Unlike traditional therapies such as chemotherapy and surgery, which can involve significant side effects and invasive procedures, the MENP approach is minimally invasive. The particles are injected into the bloodstream and guided to the tumor site by a small magnet. Upon activation by the magnetic field, they generate localized electric fields that disrupt the membranes of cancer cells, initiating natural cell death without affecting surrounding healthy tissue.
Dr. Sakhrat Khizroev, a professor in the College of Engineering and co-senior author of the study, expressed optimism about the technology, stating, “This study brings us one step closer to connecting to the human body wirelessly to help it heal in real time.” He hopes this research opens new possibilities in medicine, targeting diseases that have previously been deemed untreatable.
The study demonstrates how MENPs can be directly delivered to pancreatic tumors, where they can be remotely activated. This activation creates electric fields that differentiate between healthy and cancerous cells based on their molecular characteristics, leading to the programmed cell death of malignant cells. Dr. John Michael Bryant, a physician-scientist at Sylvester and co-senior author, noted, “Magnetoelectric nanotherapy brings a new dimension to theranostic oncology by coupling imaging and controlled physical mechanisms of tumor treatment in real time.”
MRI scans substantiated the treatment’s effectiveness, confirming reduced tumor size and generating clear imaging signals. This supports the potential of MENPs as a dual-function therapy and diagnostic tool, or “theranostic” solution. The absence of pharmaceutical drugs or biological reagents in this method minimizes side effects, suggesting it could be adapted for other challenging diseases.
The concept of using MENPs to wirelessly control local electric fields was initially proposed by Dr. Khizroev and Dr. Liang in 2011. Over the past decade, this idea has progressed through various global research collaborations and technological advancements, culminating in the current study. Despite significant progress in oncology, pancreatic ductal adenocarcinoma (PDAC) remains a major challenge, with a five-year survival rate below 10%. The disease is expected to become the second leading cause of cancer-related deaths in the United States by 2030.
Traditional treatment options, including surgery, radiation, and chemotherapy, often inflict damage on healthy tissue, while newer therapies like immunotherapy have seen limited success. One key difficulty in treating PDAC lies in the ability to control the electric fields that influence cancer cell growth; the conductive nature of human tissue complicates precise manipulation of these fields within the body. The findings from this study suggest that MENP therapy may offer a safer and more targeted treatment option for patients in the future.
Dr. Liang, co-founder of Cellular Nanomed, Inc., emphasized the potential impact of this technology: “If this technology translates successfully to humans, it could change the way we think about treating pancreatic and other solid tumors.” While the current research was conducted in preclinical models, the team believes these promising results could pave the way for future clinical trials and a new era of wireless nanomedicine.
This innovative approach to treating pancreatic cancer not only demonstrates the feasibility of targeted therapies but also raises hope for patients facing one of the most formidable challenges in cancer treatment today.
-

 Entertainment2 months ago
Entertainment2 months agoAnn Ming Reflects on ITV’s ‘I Fought the Law’ Drama
-

 Entertainment3 months ago
Entertainment3 months agoKate Garraway Sells £2 Million Home Amid Financial Struggles
-

 Health2 months ago
Health2 months agoKatie Price Faces New Health Concerns After Cancer Symptoms Resurface
-

 Entertainment2 months ago
Entertainment2 months agoCoronation Street’s Carl Webster Faces Trouble with New Affairs
-

 Entertainment2 months ago
Entertainment2 months agoWhere is Tinder Swindler Simon Leviev? Latest Updates Revealed
-

 Entertainment3 months ago
Entertainment3 months agoKim Cattrall Posts Cryptic Message After HBO’s Sequel Cancellation
-

 Entertainment2 months ago
Entertainment2 months agoOlivia Attwood Opens Up About Fallout with Former Best Friend
-

 Science2 weeks ago
Science2 weeks agoBrian Cox Addresses Claims of Alien Probe in 3I/ATLAS Discovery
-

 Entertainment3 months ago
Entertainment3 months agoMarkiplier Addresses AI Controversy During Livestream Response
-

 Entertainment2 months ago
Entertainment2 months agoMasterChef Faces Turmoil as Tom Kerridge Withdraws from Hosting Role
-

 Entertainment4 months ago
Entertainment4 months agoSpeculation Surrounds Home and Away as Cast Departures Mount
-

 World2 months ago
World2 months agoCole Palmer’s Mysterious Message to Kobbie Mainoo Sparks Speculation