Health
NHS Approves ViiV’s Apretude Injection for HIV Prevention
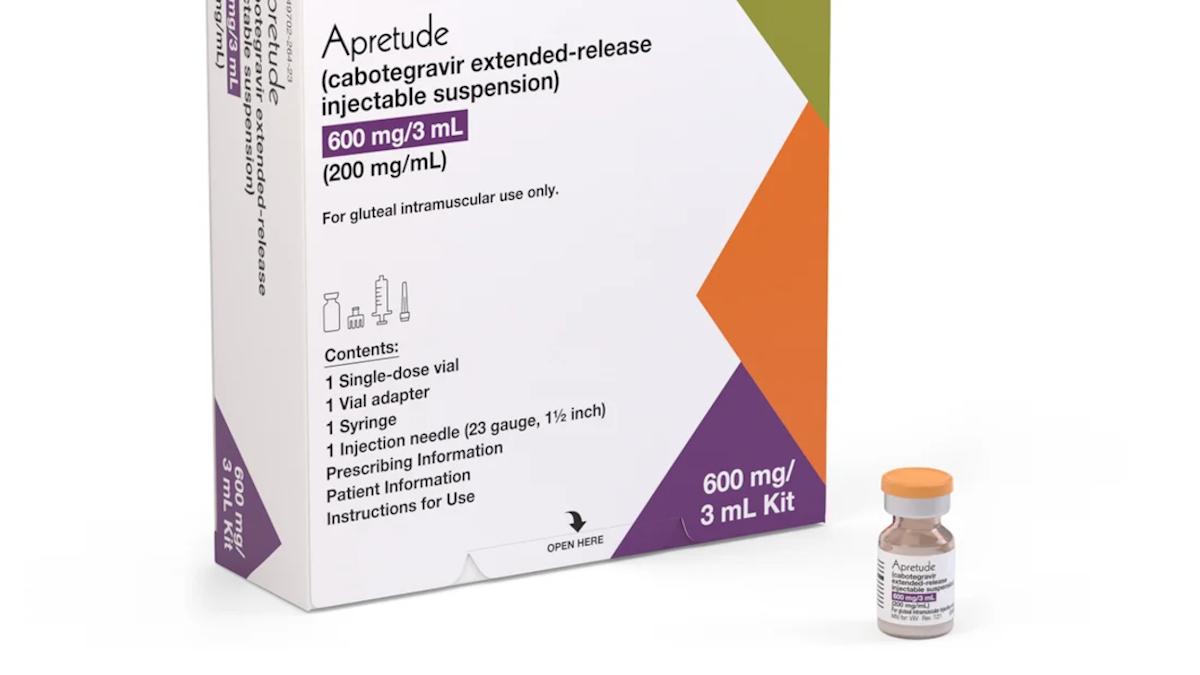
The National Health Service (NHS) in England and Wales has approved the use of an injectable form of HIV pre-exposure prophylaxis (PrEP) developed by ViiV Healthcare, a subsidiary of GlaxoSmithKline (GSK). This decision marks a significant step in addressing disparities in access to HIV prevention methods within the United Kingdom. The approval from the National Institute for Health and Care Excellence (NICE) allows for the use of Apretude (cabotegravir) as an alternative to daily oral PrEP pills, providing a new option for adults and young people at high risk of contracting HIV, particularly those with HIV-positive partners.
As of now, Apretude has been available through the NHS in Scotland since February 2024, making this approval a unifying step for the whole UK. The injectable PrEP was given the green light by the UK Medicines and Healthcare products Regulatory Agency (MHRA) in May 2024, positioning it as the first injectable option for HIV prevention in the country.
Impact on HIV Prevention Efforts
Apretude is administered as an injection every two months, which could potentially benefit around 1,000 people in England who require PrEP but are unable to take daily tablets. According to NICE, this new option fills a “critical gap” for individuals facing challenges such as contraindications to daily medication, difficulty swallowing pills, or issues related to adherence, including partner violence or homelessness.
Wes Streeting, the UK’s Health and Social Care Secretary, stated that the introduction of Apretude will support the NHS’s ambition to end new HIV cases by 2030. He highlighted that the use of PrEP has already increased by 8% this year, with over 111,000 people accessing PrEP in sexual health clinics across England in 2024, as per the latest data from the UK Health Security Agency (UKHSA). Streeting emphasized, “England will be the first country to end HIV transmissions by 2030,” describing Apretude as “another powerful tool in our arsenal.”
The rollout of Apretude is anticipated to commence within three months following the publication of NICE’s final guidance later this year. This decision has been warmly received by health advocates, including Richard Angell, chief executive of the Terrence Higgins Trust. Angell remarked that the injectable will be “transformative for our HIV response,” describing it as a vital tool in tackling inequalities and reaching those who currently do not access other forms of HIV prevention.
Future Steps and Considerations
There is also a growing call for the UK government to explore the provision of PrEP in settings beyond traditional sexual health services. This expansion could further enhance access and utilization among populations that may face barriers to care.
While another injectable option, developed by Gilead Sciences and known as lenacapavir, has been approved in the United States and European Union, it has not yet been submitted for approval in the UK. A submission is expected later this year, which could provide further options for individuals seeking HIV prevention.
As the NHS moves forward with this new treatment option, the focus remains on ensuring equitable access to HIV prevention strategies, ultimately aiming for a significant reduction in new HIV infections across the UK.
-

 Entertainment2 months ago
Entertainment2 months agoAnn Ming Reflects on ITV’s ‘I Fought the Law’ Drama
-

 Entertainment3 months ago
Entertainment3 months agoKate Garraway Sells £2 Million Home Amid Financial Struggles
-

 Entertainment2 months ago
Entertainment2 months agoCoronation Street’s Carl Webster Faces Trouble with New Affairs
-

 Health1 month ago
Health1 month agoKatie Price Faces New Health Concerns After Cancer Symptoms Resurface
-

 Entertainment1 month ago
Entertainment1 month agoWhere is Tinder Swindler Simon Leviev? Latest Updates Revealed
-

 Entertainment3 months ago
Entertainment3 months agoKim Cattrall Posts Cryptic Message After HBO’s Sequel Cancellation
-

 Entertainment2 months ago
Entertainment2 months agoMasterChef Faces Turmoil as Tom Kerridge Withdraws from Hosting Role
-

 Entertainment3 months ago
Entertainment3 months agoSpeculation Surrounds Home and Away as Cast Departures Mount
-

 World1 month ago
World1 month agoCole Palmer’s Mysterious Message to Kobbie Mainoo Sparks Speculation
-

 Entertainment2 months ago
Entertainment2 months agoITV’s I Fought the Law: Unraveling the True Story Behind the Drama
-

 Entertainment1 month ago
Entertainment1 month agoCaz Crowned Winner of The Great British Sewing Bee, Overjoyed by Triumph
-

 Entertainment3 months ago
Entertainment3 months agoMarkiplier Addresses AI Controversy During Livestream Response